Mesenchymal stem cells are multipotent stem cells with the capacity for both self-renewal and differentiation. They can transform into various cell types, offering a wide range of clinical applications. MSCs are present in various sources, including the umbilical cord, dental pulp, adipose tissue (fat), and bone marrow.
MSCs operate through the secretion of bioactive factors and exosomes using the endocrine and paracrine systems. They release various molecules, including cytokines, growth factors, antioxidants, pro-angiogenic factors, and factors that stimulate cell proliferation and angiogenesis. These secretions mitigate stress responses and apoptosis in damaged cells, facilitating tissue repair. Additionally, MSCs play a role in the regulation of local and systemic inflammatory and immune responses, contributing to their therapeutic effects.
- Homing functionMSCs exhibit a unique homing function. When body tissues are stimulated, they activate previously dormant cells, prompting them to migrate to the site of injury. MSCs play a crucial role in regulating the migration of stem cells to specific tissues through various signalling pathways, facilitating the repair of damaged or ageing tissues in the human body.

- Anti-inflammatory effectMSCs possess innate immunogenic advantages that make them effective in reducing Graft-versus-host disease (GvHD). They can modulate the immune system through direct contact and the release of soluble cytoprotective factors, influencing immune cells in the body and promoting anti-inflammatory responses.
- Good secretion of cytokinesMSCs exhibit strong migratory capacity, differentiation potential, and the secretion of various beneficial cytokines. These cytokines can stimulate MSCs to engage in immunosuppressive functions and rapidly generate a substantial number of good stem cells. This process promotes the survival and regeneration of damaged cells.

- Promote angiogenesisAngiogenesis is a finely tuned process influenced by a delicate balance of pro-angiogenic and anti-angiogenic factors. MSCs have the capacity to initiate and enhance angiogenesis, which is why they are often regarded as the preferred cells for tissue regeneration. They have been successfully employed in the treatment of conditions such as skin defects, ischemic diseases, and nerve damage, harnessing their angiogenic potential for therapeutic benefits.
MSCs possess the capability to modulate a patient’s immune system, stimulate cell growth, and differentiate into diverse specialised cell types. Their potential applications have extended to a broad spectrum of diseases, including but not limited to heart disease, diabetes, Alzheimer’s disease, spinal cord injury, chronic trauma, Graft-versus-host disease, rheumatoid arthritis, liver cirrhosis, retinal diseases, and numerous others.
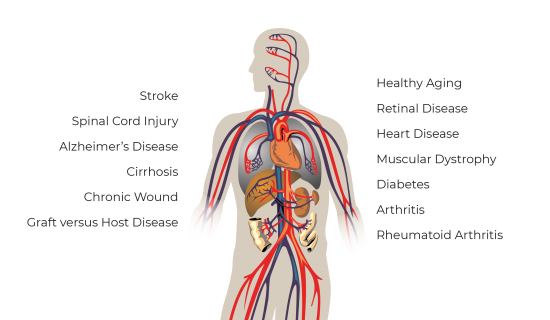
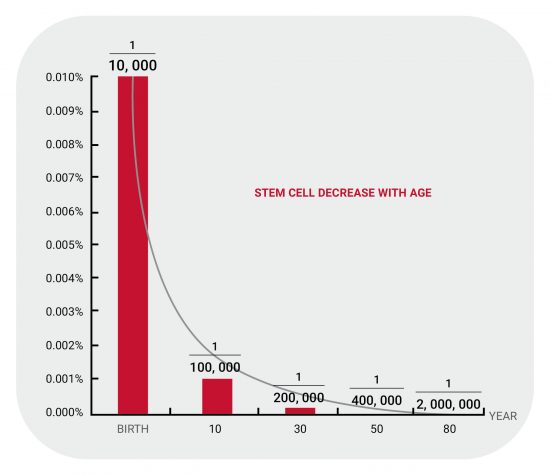
Research has indicated that replenishing an adequate quantity of stem cells within the body can trigger other endogenous stem cells found in the human body. This, in turn, enhances the metabolic functions of cells and organs, ultimately leading to a potential delay in the overall ageing process.
The potential benefits encompass a wide range of improvements, including increased energy and physical vitality, enhanced overall health, anti-ageing effects, heightened libido, restoration of hormonal balance, firmer and more youthful skin, and improvements in memory.
- Youth and Primitiveness
MSCs sourced from the umbilical cord are the youngest and most primitive type of MSCs found in the human body. Their relative youth makes them highly potent and effective.
- Ease of Multiplication:
Umbilical cord MSCs can be readily multiplied in laboratory settings through cell culture, providing an abundant and accessible source for various medical applications.
- Immunoprivileged
These MSCs possess immunoprivileged properties, meaning they are less likely to trigger an immune response when transplanted into recipients, increasing their compatibility and suitability for therapeutic use.
- Aid in Hematopoietic Stem Cell (HSC) Transplants
Umbilical cord-derived MSCs play a valuable role in the engraftment process when HSC transplants are performed, enhancing the success of the transplant.
- Abundant Wharton’s Jelly
The Wharton’s Jelly within the umbilical cord contains a particularly rich concentration of MSCs compared to other sources like bone marrow and fat tissue, making it a highly attractive source of these vital stem cells. This abundance further enhances their potential for a wide range of medical applications.
| Content Of Mesenchymal stem cells per 200 million [Nucleated cells] | |
|---|---|
| Cord Blood | 1 cell |
| Adult Bone Marrow | 2,000 cells |
| Wharton’s Jelly | 666,000 cells |
*Reference: BMC Cell Biology 2006,7: 14
- Ageing of MSCs
As individuals age, MSCs also experience ageing, leading to a reduction in both their quantity and functionality. This ageing process is associated with reduced regenerative potential.
- Apoptosis
As MSCs age, some of them may undergo apoptosis, a programmed cell death process. This can contribute to a decrease in the total number of functional MSCs in the body.
- Impact on Skin Ageing
The decline in both the quantity and functionality of MSCs can have an impact on various aspects of ageing, including skin ageing. Reduced regenerative capacity may result in the development of wrinkles, loss of skin elasticity, and other age-related skin changes.
Clinical research on MSCs is on the rise, with significant trends and observations. The worldwide landscape of MSC clinical studies is notable. China, Europe, and the United States are at the forefront of clinical research in this field, indicating its global importance and widespread interest.
MSCs hold promise for the treatment of a wide array of diseases. Clinical research encompasses hundreds of conditions, with particular emphasis on three primary areas: nervous system, cardiovascular, and orthopaedic diseases.
In addition to the core areas, clinical research on MSCs extends to various other diseases, including diabetes, liver disorders, lung diseases, gastrointestinal tract conditions, skin ailments, and Graft-versus-host disease (GvHD). This diversification underscores the versatility and potential of MSCs in addressing a wide range of medical challenges.
The data from Clinicaltrials.gov reflects the growing interest and investment in MSC research and its application in addressing diverse health issues. This suggests that MSCs will continue to play a pivotal role in future medical advancements, with an expanding range of therapeutic applications.
| No. | Product Name | Company | Approved Country | Regulator | Year | Indications | Product Type |
|---|---|---|---|---|---|---|---|
| 1 | Queencell | Anterogen Co. Ltd. | South Korea | MFDS (KFDA) | 2010 | Subcutaneous tissue defects | Autologous human AT-MSC |
| 2 | Cellgram-AMI | Pharmicell Co. Ltd. | South Korea | MFDS (KFDA) | 2011 | Acute myocardial infarction | Autologous human BM-MSC |
| 3 | Cartistem | Medipost Co. Ltd. | South Korea | MFDS (KFDA) | 2012 | Knee articular cartilage defects | Allogeneic human UC-MSC |
| 4 | Cupistem | Anterogen Co. Ltd. | South Korea | MFDS (KFDA) | 2012 | Crohn’s fistula | Autologous human BM-MSC |
| 5 | Remestemcel-L | Osiris Therapeutics Inc., Mesoblast Ltd. | Canada, New Zealand |
Health Canada, MedSafe | 2012 | GvHD | Allogeneic human BM-MSC |
| 6 | Neuronata-R | Corestem Inc. | South Korea | MFDS (KFDA) | 2014 | Amyotrophic lateral sclerosis | Autologous human BM-MSC |
| 7 | Temcell HS | JCR Pharmaceuticals | Japan | PMDA | 2015 | GvHD | Allogeneic human BM-MSC |
| 8 | Holoclar | Chiesi Farmaceutici | Europe | EMA | 2015 | Limbal stem cell deficiency caused by burns | Autologous human Limbal MSC |
| 9 | Astrostem | Nature Cell Co. Ltd | Japan | MHLW | 2015 | Autoimmune disease | Allogeneic human AD-MSC |
| 10 | Stempeucel | Stempeutics Research PVT | India | CDSCO | 2016 | Critical limb ischemia | Allogeneic human BM-MSC |
| 11 | Alofisel | TiGenix NV/Takeda | Europe, Japan | EMA | 2018 | Complex perianal fistulas in Crohn’s disease | Allogeneic human AT-MSC |
| 12 | Stemirac | Nipro Corp | Japan | PMDA | 2018 | Spinal cord injury | Autologous human BM-MSC |
| 13 | Mesestro-Cell | Cell Tech Pharmed (Iran) | Iran | Iran FDA | 2018 | Osteoarthritis | Autologous human BM-MSC |
| 14 | Ryoncil | Mesoblast Limited | United States | US FDA | 2024 | Steroid-refractory acute GvHD | Allogeneic human BM-MSC |
| 15 | Amimestrocel | Platinumlife Biotechnology (Beijing) Co. Ltd | China | NMPA | 2025 | Steroid-refractory acute GvHD | Allogeneic human UC-MSC |
| No. | Product Name |
|---|---|
| 1 | Queencell |
| 2 | Cellgram-AMI |
| 3 | Cartistem |
| 4 | Cupistem |
| 5 | Remestemcel-L |
| 6 | Neuronata-R |
| 7 | Temcell HS |
| 8 | Holoclar |
| 9 | Astrostem |
| 10 | Stempeucel |
| 11 | Alofisel |
| 12 | Stemirac |
| 13 | Mesestro-Cell |
| 14 | Ryoncil |
| 15 | Amimestrocel |
| Company | Approved Country | Regulator | Year | Indications | Product Type |
|---|---|---|---|---|---|
| Anterogen Co. Ltd. | South Korea | MFDS (KFDA) | 2010 | Subcutaneous tissue defects | Autologous human AT-MSC |
| Pharmicell Co. Ltd. | South Korea | MFDS (KFDA) | 2011 | Acute myocardial infarction | Autologous human BM-MSC |
| Medipost Co. Ltd. | South Korea | MFDS (KFDA) | 2012 | Knee articular cartilage defects | Allogeneic human UC-MSC |
| Anterogen Co. Ltd. | South Korea | MFDS (KFDA) | 2012 | Crohn’s fistula | Autologous human BM-MSC |
| Osiris Therapeutics Inc., Mesoblast Ltd. | Canada, New Zealand | Health Canada, MedSafe | 2012 | GvHD | Allogeneic human BM-MSC |
| Corestem Inc. | South Korea | MFDS (KFDA) | 2014 | Amyotrophic lateral sclerosis | Autologous human BM-MSC |
| JCR Pharmaceuticals | Japan | PMDA | 2015 | GvHD | Allogeneic human BM-MSC |
| Chiesi Farmaceutici | Europe | EMA | 2015 | Limbal stem cell deficiency caused by burns | Autologous human Limbal MSC |
| Nature Cell Co. Ltd | Japan | MHLW | 2015 | Autoimmune disease | Allogeneic human AD-MSC |
| Stempeutics Research PVT | India | CDSCO | 2016 | Critical limb ischemia | Allogeneic human BM-MSC |
| TiGenix NV/Takeda | Europe, Japan | EMA | 2018 | Complex perianal fistulas in Crohn’s disease | Allogeneic human AT-MSC |
| Nipro Corp | Japan | PMDA | 2018 | Spinal cord injury | Autologous human BM-MSC |
| Cell Tech Pharmed (Iran) | Iran | Iran FDA | 2018 | Osteoarthritis | Autologous human BM-MSC |
| Mesoblast Limited | United States | US FDA | 2024 | Steroid-refractory acute GvHD | Allogeneic human BM-MSC |
| Platinumlife Biotechnology (Beijing) Co. Ltd | China | NMPA | 2025 | Steroid-refractory acute GvHD | Allogeneic human UC-MSC |